ISO 13485 (Quality Management System for Medical Device) &
GDPMD (Good Distribution Practice for Medical Device)
GDPMD (Good Distribution Practice for Medical Device)
Safeguarding Medical Device, Ensuring Safety at Every Stage
ISO 13485 (Quality Management System for Medical Device) &
GDPMD (Good Distribution Practice for Medical Device)
GDPMD (Good Distribution Practice for Medical Device)
Safeguarding Medical Device, Ensuring Safety at Every Stage

What is ISO 13485 ?
ISO 13485 and GDPMD are two essential standards within the medical device industry, each focusing on different aspects of ensuring product quality and safety. ISO 13485 establishes requirements for a comprehensive quality management system, covering all stages of the product lifecycle from design to distribution. It ensures that medical device manufacturers and suppliers adhere to international standards, effectively manage risks, and continuously improve processes to enhance product quality and customer satisfaction.

What is ISO 13485 ?
ISO 13485 and GDPMD are two essential standards within the medical device industry, each focusing on different aspects of ensuring product quality and safety. ISO 13485 establishes requirements for a comprehensive quality management system, covering all stages of the product lifecycle from design to distribution. It ensures that medical device manufacturers and suppliers adhere to international standards, effectively manage risks, and continuously improve processes to enhance product quality and customer satisfaction.
On the other hand, GDPMD provides guidelines for the proper storage, transportation, and handling of medical devices during distribution, ensuring their integrity and effectiveness are maintained throughout the supply chain. Compliance with GDPMD minimizes risks associated with product damage, contamination, or deterioration during transportation and storage, ultimately safeguarding patient safety and regulatory compliance.
Together, ISO 13485 and GDPMD play crucial roles in maintaining high standards of quality and safety within the medical device industry, contributing to improved patient outcomes and public health.
On the other hand, GDPMD provides guidelines for the proper storage, transportation, and handling of medical devices during distribution, ensuring their integrity and effectiveness are maintained throughout the supply chain. Compliance with GDPMD minimizes risks associated with product damage, contamination, or deterioration during transportation and storage, ultimately safeguarding patient safety and regulatory compliance.
Together, ISO 13485 and GDPMD play crucial roles in maintaining high standards of quality and safety within the medical device industry, contributing to improved patient outcomes and public health.

What are the Differences Between ISO 13485 & GDPMD?
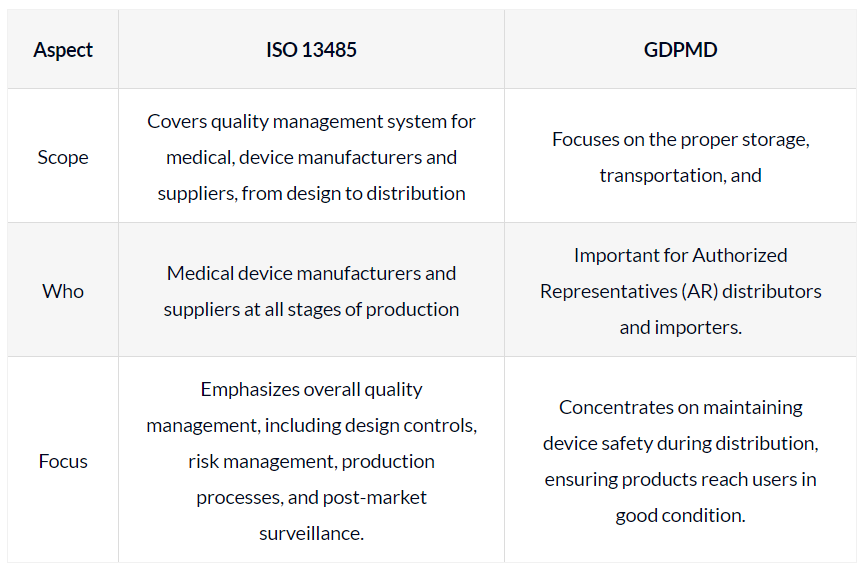
| Aspect | ISO 13485 | GDPMD |
|---|---|---|
| Scope | Covers quality management system for medical, device manufacturers and suppliers, from design to distribution | Focuses on the proper storage, transportation, and |
| Who | Medical device manufacturers and suppliers at all stages of production | Important for Authorized Representatives (AR) distributors and importers. |
| Focus | Emphasizes overall quality management, including design controls, risk management, production processes, and post-market surveillance. | Concentrates on maintaining device safety during distribution, ensuring products reach users in good condition. |
What are the Differences Between ISO 13485 & GDPMD?
Leave the heavy lifting to us- we’ve got you covered
How We Guide

Discovery Session
Schedule a complimentary private consultation with our consultant to explore your needs and expectations.
Gap Analysis & Awareness Building
We identify organizational gaps and enhance team awareness, ensuring sustainable system maintenance post-certification
Documentation Preparation & Ensuring Compliance
We tailor documentation to your business needs ensures a seamless transition and conduct thorough internal audits to ensure your ISO 13485 & GDPMD meets global standards
You are ISO certified!
Showcase your certification and impress your client!
Real Experiences
Trusted by Our Amazing Clients

























Comprehensive Answers to Your ISO 13485 & GDPMD Certification Queries
Your ISO 13485 & GDPMD Questions Answered
Comprehensive Answers to Your ISO 13485 & GDPMD Certification Queries


